 Add My Company
Add My Company
Introduction to Reverse Engineering
Reverse engineering means taking a device apart to understand how it works. It involves creating digital models that mimic the original design. This process differs from creating new devices because you're copying or improving existing ones. Legal concerns like patents must be considered to avoid infringing on rights.
Reverse engineering in orthopedics must follow ethical standards. Only proceed when authorised to avoid legal trouble. Proper planning and documentation are critical at every step.
Step-by-Step Approach to Reverse Engineering Orthopedic Devices.
Assessment: Examine the device carefully. Record its features, functions, and materials used.
Disassembly: Carefully take parts apart, noting how each piece fits and functions.
Data Collection: Measure parts precisely. Identify materials and test their strength or flexibility.
Modelling: Use CAD software to create a digital replica of the device.
Validation: Test the reverse-engineered part to ensure it matches the original’s performance and safety.
Tools and Technologies Used
3D Scanning: Capture detailed shapes and features quickly.
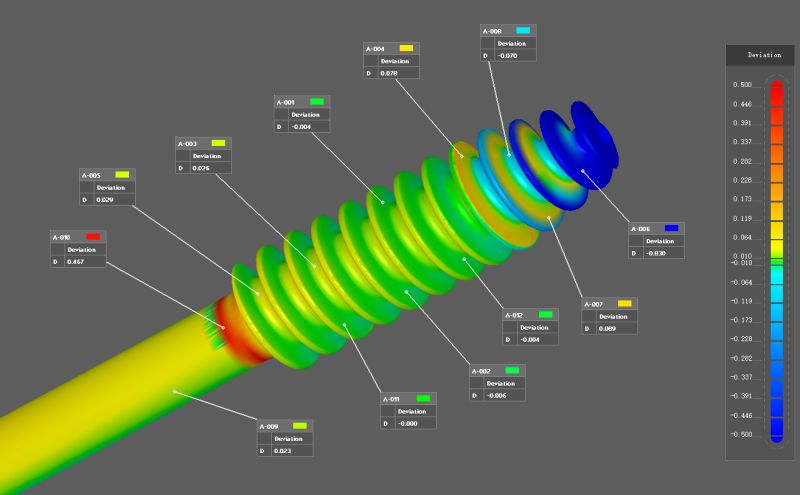
CAD/CAM Software: Design and simulate new or replacement parts. Validation using software such as verisurf.
Material Analysis Tools: Check what materials are safe and effective.
For more information on CereScan 3D Laser ScanneA legacy project explaining the Process of Reverse Engineering in Orthopedics talk to 3D Scanning Solutions
