 Add My Company
Add My Company

Freeze drying has become a popular technique across a variety of industries including, but not limited to diagnostics, pharmaceutical, biotech, and food to name a few, all of whom use freeze drying in the processing of their products in different ways.
Why are the parameters of cycle development variable?
Before a sample can be freeze dried (lyophilised), the freeze drying cycle must be carefully designed to produce optimum conditions for the final product. As consumers and buyers of freeze-dried goods, we often take this for granted but there is a lot that is taken into consideration and occurs behind the scenes to meet the product quality attributes in terms of product stability and functionality.
A commonly occurring misconception for cycle design and development we have heard numerous times is that “This cycle works well for this product; it can be applied to other products we have’”. Unfortunately, this is rarely, if ever, the case, so we cannot emphasise enough the importance of the role of designing lyo-cycle parameters during cycle development that are suitable for your desired end-product.
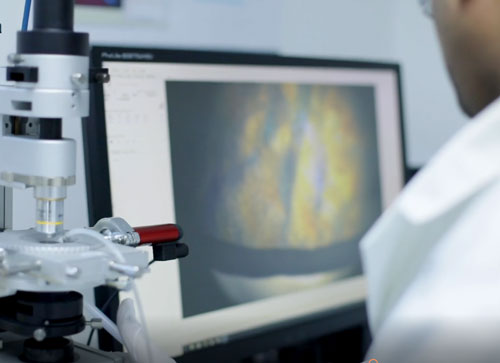
At Biopharma Group, dedicated in-house project managers and lyo scientists communicate and collaborate with our clients throughout the development and processing stages of a project from start to finish, to produce the best possible conditions for developing an effective freeze drying cycle.
The process begins by determining the critical temperatures specific to client’s sample/s using the pre-lyophilised material. These parameters are obtained using analytical lyo instrumentation including the Lyostat (freeze drying microscope), Lyotherm (combination of differential thermal analysis and electrical impedance) and frozen state (pre-lyo) Differential Scanning Calorimetry (DSC) analysis. Based on the results of the initial thermal characterisation, a series of developmental cycles with associated post process analyses to fine tune the process parameters, followed by a confirmatory cycle to evaluate the process repeatability.
The benefits of an advanced and fully developed freeze drying cycle by Quality by Design (QbD) include:
- The removal of a “trial and error” approach, which removes batch failures/ product rejection and reduces the overall processing and production costs
- Time saving, especially when the lyo process is transferred and scaled up.
- An increase in product stability and shelf life
- Increase in acceptable storage temperature, leading to a reduction in costs, since the cold chain is removed.
- Improved appearance and robustness of freeze-dried cake or lyobead
- Faster reconstitution times.
To discuss your cycle development requirements, please get in touch HERE
For more information on The importance of cycle parameters in lyophilisation & why one size does not fit all talk to Biopharma Group

